International studies run by CROs are essential to the pharmaceutical industry, but the high level of regulation required by multiple governing bodies makes running a clinical trial a complex undertaking.
Added to this is the challenge of reproducing studies in different countries and languages while maintaining a high level of accuracy and conformity.
Translation for CROs requires the attention of a dedicated team of linguists who have specialist knowledge and experience of working with the pharmaceutical industry. Choosing the right translation agency is key to the success of your international clinical trial - high quality medical translations can quite literally save lives.
Here are five tips for translation in the life sciences sector:
1. Prepare a detailed brief
Briefs for CRO translation projects are often not given enough attention, generally because of a lack of time or because their importance is underestimated. A detailed brief, however, allows a translation project to run more smoothly. Understanding the context for which they are translating helps linguists to make the best decisions in their work, whether in choice of vocabulary or formatting.
It is particularly important for a translator to know which audience they are translating for – will the document be patient-facing or for use by medical professionals? This will have an impact on whether a medical term is used, such as ‘myalgia’, or a layman’s term, such as ‘muscle pain’.
It can also be helpful for a translator to understand exactly what the document will be used for once they have finished with it, if the CRO is able to provide this information.
2. Use a glossary of specific terms
Glossaries are very useful for medical translators to ensure a high level of accuracy. This is clearly very important in clinical studies, where a technical term may seem very similar to another, but in fact has a crucial difference in its meaning or application. The use of a glossary also guarantees consistency throughout a text, particularly when multiple translators are working on the same translation project.
Glossaries can include:
| Examples | ||
|
Technical/medical terms |
Hypertension (French) |
Hypertension (English – medical term) High blood pressure (English – layman's term) |
|
Acronyms and abbreviations |
EKG (Elektrokardiogramm) (German) | ECG or EKG (electrocardiogram) (English) |
| Proper nouns | La OMS (Organización Mundial de la Salud) (Spanish) | WHO (World Health Organization) (English) |
| Style guide | Phase IV vs. Phase 4 | |
3. Check translations using ‘back translation’
A technique called back translation is used during the process of translating documents for clinical studies. First of all, the text is translated into the target language. Next, this translation is translated back into the original (source) language by an independent linguist who has not seen the original document.
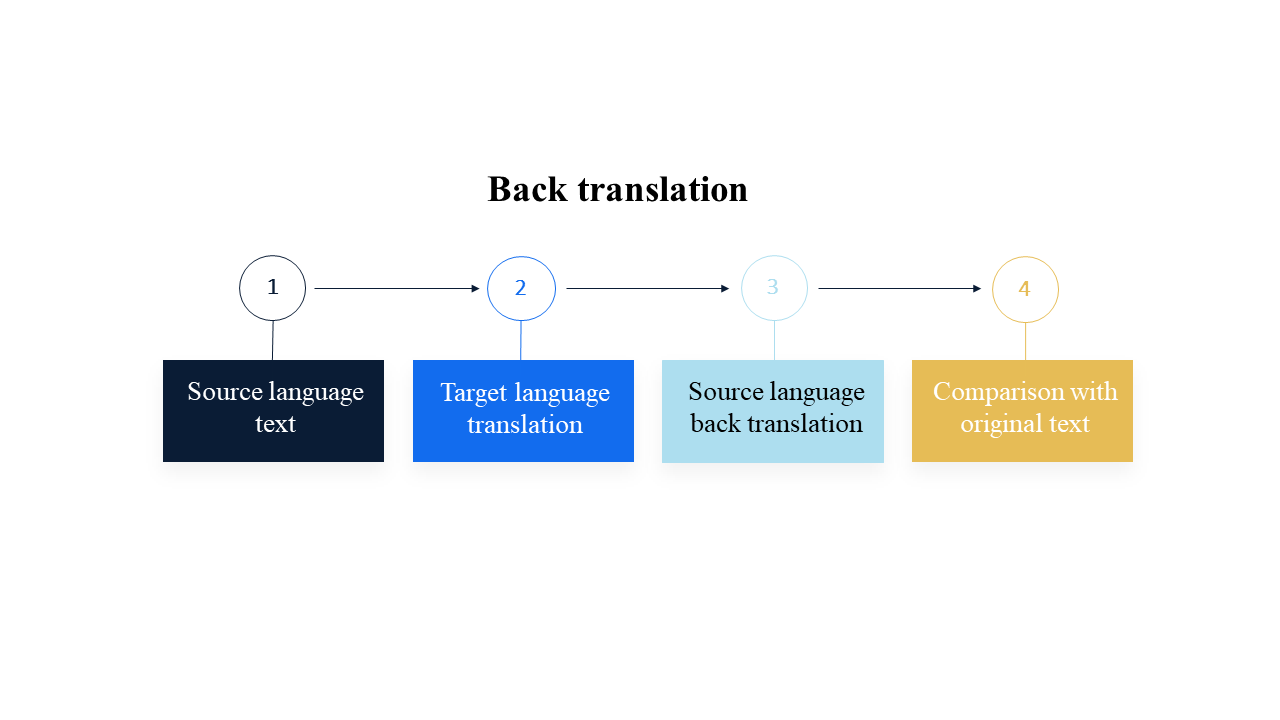 The back translation is compared with the original text and may be used to display the nuances in meaning (e.g. for a client who is not familiar with the target language) or to identify any discrepancies in meaning or tone.
The back translation is compared with the original text and may be used to display the nuances in meaning (e.g. for a client who is not familiar with the target language) or to identify any discrepancies in meaning or tone.
This process is particularly important in medical translation, where the slightest mistranslation could have serious consequences. Because of the need for perfect accuracy, back-translation is often used as part of a longer process called linguistic validation.
4. Confirm instrument validity using ‘linguistic validation’
Linguistic validation is used to ensure that all participants understand the exact same meaning and tone in a document that has been translated into multiple languages for the same study. It is therefore an important part of checking the validity of an instrument, such as a questionnaire for use in PROs.
The process involves several steps:

Without linguistic validation, there is a risk of bias in the results of a study, due to the increased likelihood of patients in different countries interpreting questions in different ways. In the same way that great care is taken over a physical instrument used in a trial, this thorough process is very important to make sure that text-based instruments are highly precise and accurate.
The last part of linguistic validation is common to all translations…
5. Proofread all work carefully
An important final step in any translation project is careful proofreading to check for errors, such as spelling and grammar, and any inconsistencies or issues with formatting.
Expert linguists work on translations in their native language to ensure that the text is clear and that the style and tone suit the context. They compare the translation with the original text to check that all details have been correctly translated.
The task of proofreading requires high attention to detail and good knowledge of the subject matter, as well as the necessary language skills.
Do you need a translation partner for your international clinical studies? Download our free e-book, ‘Best practices for translation in the pharmaceutical industry’.

